Cystoid Macular Edema (CME): Managing Post-Cataract Surgical Complications
Cystoid Macular Edema is a condition of the retina in which fluid builds up in the macula central area responsible for clear central vision.
This condition involves ocular inflammation precipitated by cataract surgery, known as pseudophakic CME. It reported incidence after cataract surgery a common complication of 0.1-2.35%.
CME is the most common cause of decreased vision in patients following complicated cataract surgery, occurring much more frequently than either retinal detachment or endophthalmitis. Although CME was clinically recognized and described over 50 years ago much remains unknown about it.
Patients after cataract surgery are at risk for developing CME, a common complicated surgery leading to reduced vision. There are certain demographics that are considered a higher risk, including patients with diabetes, diabetic retinopathy, uveitis, posterior capsule rupture, vitreous prolapse, and previous retinal vein occlusions. Male gender and old age have also been identified as risk factors.
Pathophysiology of Macular Edema
Inflammation plays a large role in the pathogenesis of CME. Pro-inflammatory mediators substances such as Nitric oxide, Vascular Endothelial Growth Factor (anti-VEGF injections), Prostaglandins, cytokines, and other mediators are involved in the inflammatory process that can occur following modern cataract surgery. This inflammation process leads to the destruction of the blood-retinal barrier causing increased vascular permeability. This results in edema of the inner nuclear layer, edema of the outer plexiform layer, accumulation of sub-retinal fluid and ultimately thickening of the retina.
The precise pathophysiology of CME has yet to be fully understood, several factors have been implicated in its development, including vascular instability, vascular traction, and relative ocular hypotony.
CME can lead to permanent vision loss, even after the edema resolves. This is believed to occur due to structural changes in the photoreceptors, which are more prevalent in chronic cases of CME.
Evaluation & Diagnosis
There are different methods for evaluating CME including non-contact and contact slip lamp biomicroscopy, FFA, Fundus stereo photography, indirect ophthalmoscopy, and OCT. Currently, FFA and OCT are the most used investigative tools.
Slit-lamp Biomicroscopy
They are typically conducted using a 78D lens and a 90D lens in the initial step to evaluate macular edema. This microscopic evaluation method reveals the location and presence of macular thickness, exudates, and cystoid changes.
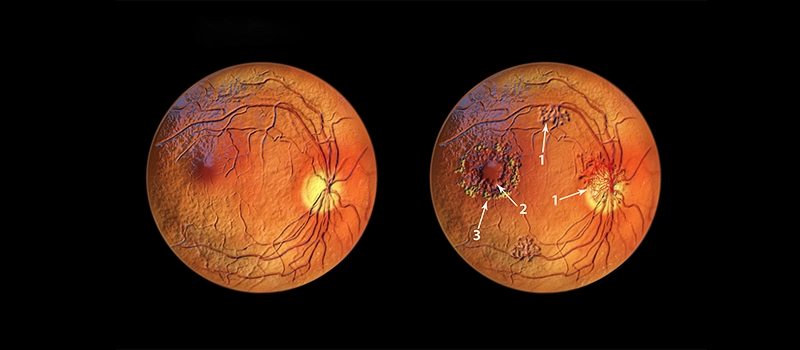
CME is characterized by a unique stellate or radially oriented pattern in the peri-foveal thickness of cysts, attributed to the oblique arrangement of the Henle fiber layer.
Outside the macular region, edema presents a honeycomb appearance caused by the perpendicular alignment of the outer plexiform layer. A central cyst linked to CME may resemble a macular thickness. However, performing the Watzke-Allen test using slit lamp biomicroscopy with a 90D lens reveals an intact vertical without a central break.
Fundus Fluorescein Angiography
FFA can identify areas of retinal capillary leakage. The Early Treatment Diabetic Retinopathy Study (ETDRS) classifies diabetic macular edema into diffuse focal types based on the extent of fluorescein leakage associated with microaneurysms.
According to ETDRS criteria, focal diabetic edema shows 67% or more leakage linked to microaneurysms, intermediate exhibits 33% TO 66% leakage, and diffuse displays less than 33% leakage associated with microaneurysms.
In the early phase of fluorescein angiography, choroidal fluorescence may be partially obscured by significant edema, whether cystoid or non-cystoid especially if the edema is turbid due to lipid-laden macrophages. Dilation of the fine capillary network of telangiectatic retinal vessels around the fovea may be evident in the arteriovenous phase.
In late-phase imaging, hyperfluorescence results from dye leakage from retinal vessels, influenced by the extent of dysfunction in the retinal vascular endothelium. This hyperfluorescence can appear as cystic or irregular staining, filling cystoid spaces rapidly with pronounced leakage or appearing later if leakage is less significant.
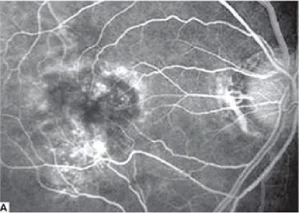
Fig.1.A: Fluorescein angiography shows cystoid macular edema.

Fig.1.B: Clinical photograph shows vitreous incarceration into the wound.
Fig.1.C: Clinical photograph post-vitreolysis.
Cystoid Macular Edema Optical Coherence Tomography (OCT)
Clinicians utilize OCT to assess macular edema stemming from conditions such as age-related macular degeneration, diabetic retinopathy, hereditary retinal degeneration, retinal vein occlusion, edema after cataract surgery, epiretinal membrane, and history of uveitis. Due to its high reproducibility, OCT has emerged as the preferred diagnostic tool for diagnosing and monitoring CME. OCT allows clinicians to identify, locate, and measure fluid accumulations, enabling accurate assessments and ongoing monitoring.
Furthermore, OCT’s ability to categorize different diseases supports prognosis, assists in disease management, predicts patient outcomes, and aids in treatment planning.
- Diabetic Macular Edema: Various patterns of fluid accumulation are visible on OCT scans in patients with diabetic macular edema.
- Diffuse Retinal Thickening: It is defined by retinal thickening exceeding 200um in height and width, featuring regions of reduced reflectivity, particularly noticeable in the outer retinal layers.
- CME: It is characterized by intraretinal fluid accumulation within well-defined spaces of low reflectivity typically around the outer plexiform layer but involving the photoreceptor and inner retinal layers.
- Posterior Hyaloid Traction or Taut Posterior Hyaloid Membrane: This is identified by the presence of a highly reflective membrane on the inner retinal surface, which causes traction and elevation of the retina.
- Subretinal Fluid: It is identified as a dome-shaped dark area situated between the neurosensory retina and the retinal pigment epithelium.
- Tractional Retinal Detachment: This is identified by a peak-shaped retinal detachment caused by traction from proliferative membranes on the retinal surface or within the vitreous. This condition appears as a low signal area beneath the highly reflective border of the detached retina.
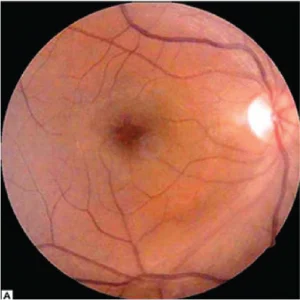
Fig 2.A Color fundus photograph shoes postoperative cystoid macular edema.
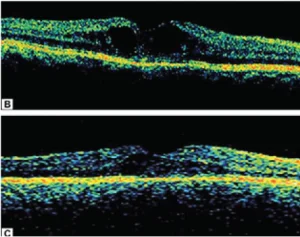
Fig. 2.B Optical coherence tomography shows increased macular thickness with cystoid cavities.
Fig.2. C. Optical coherence tomography shows marked resolution of cystoid space with decrease in macular thickness following sub-Tenon triamcinolone injection.
Radiation Retinopathy
OCT allows clinicians to assess radiation retinopathy using a 5-point grading system, which correlates with visual acuity.
- Grade 1: Foveola-sparing non-cystoid macular edema.
- Grade 2: Foveola-sparing cystoid macular edema.
- Grade 3: Foveola-involving non-cystoid macular edema.
- Grade 4: Mild-to-moderate foveola-involving cystoid macular edema.
- Grade 5: Foveola-involving severe cystoid macular edema.
Juvenile X-linked Retinoschisis
It classifies juvenile X-linked retinoschisis into different types in OCT findings:
- Type 1 or Foveal: Absence of both lamellar schisis on OCT and peripheral schisis on the ophthalmoscopy.
- Type 2 or Foveolamellar: Presence of lamellar schisis on OCT without peripheral schisis on the ophthalmoscopy.
- Type 3 or Complex: Lamellar schisis on OCT and peripheral schisis on the ophthalmoscopy.
- Type 4 or Foveoperipheral: Presence of peripheral schisis on the ophthalmoscopy.
The finding of juvenile X-linked retinoschisis is the presence of a spoke-wheel pattern in the macula, observable in high magnification in patients ages 30 or younger.
On OCT patients with uveitis exhibit diffuse macular edema, CME, and subretinal detachment.
Treatment for Cystoid Macular Edema (CME)
A stepwise medical treatment approach is crucial for managing CME, which involves systemic and ocular pharmaceutical agents. Surgical procedures may also be needed in certain cases.
Systemic Therapy
Many patients develop macular edema as a secondary manifestation of other health conditions such as hypertension, diabetes, dyslipidemia, or inflammatory conditions, it is essential to address these underlying systemic issues.
Some research shows that strict glycemic control can be effective in delaying the onset and progression of diabetic retinopathy in both type 1 and type 2 diabetes.
Intravitreal Anti-Vascular Endothelial Growth Factor
The primary approach for macular edema across various pathologies is intravitreal injections of anti-VEGF agents. Clinicians currently use three formulations of intravitreal anti-VEGF therapies.
Bevacizumab is a 148-kDa humanized full-size monoclonal IgG1 antibody that targets all subtypes of VEGF-A, available in a concentration of 1.25mg/0.05mL.
Ranibizumab offered in concentrations of 0.3mg/0.05mL and 0.5mg/0.05mL, is a 48-kDa humanized monoclonal antibody fragment that targets all subtypes of VEGF-A.
Aflibercept, provided in a concentration of 2mg/0.05mL, is a 115-kDa fusion protein that targets VEGF-A, VEGF-B, and placental growth factors.
Ocular Topical Treatment
Nonsteroidal Anti-inflammatory Drugs
Using topical steroids and NSAIDs prevents prostaglandin synthesis by inhibiting the COX enzyme. Common ocular side effects of topical NSAIDs include burning, stinging, and conjunctival redness. Clinicians should also consider steroid-related adverse effects, such as increased intraocular pressure (IOP), delayed wound healing, and susceptibility to infections.
The latest treatment algorithm for CME, developed by the Jampol lab, involves a combination treatment of typical NSAIDs with topical corticosteroids, such as diclofenac and fluorometholone. If there is no improvement in duration after treatment of 4-6 weeks, an alternative NSAIDS like nepafenac or bromfenac may be prescribed. If vision does not improve after 4-6 weeks of nepafenac or bromfenac treatment, consideration may be given to using an intravitreal corticosteroid injection. Additionally, for cases of treatment-resistant CME, subtenon triamcinolone should be considered as a therapeutic option.
Surgical Treatment for Macular Edema
Vitrectomy is frequently performed for macular edema caused by epiretinal membrane (ERM) thickening of the macula. It is less commonly done for macular edema unless it is related to vitreomacular traction syndromes or taut posterior hyaloid face (TPHC) syndrome, which is often associated with diabetic retinopathy.
Conclusion
Cystoid Macular Edema (CME) is a common complication following cataract surgery, leading to vision impairment if not managed properly. CME occurs due to the accumulation of fluid in the macula and requires prompt diagnosis and treatment, which may include steroid therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), and surgical interventions. For MBBS students and clinicians studying ophthalmology, DigiNEET offers in-depth video lectures on the pathophysiology, clinical presentation, and treatment options for post-cataract complications like CME. The platform also features interactive MCQs, case studies, and mock exams that focus on high-yield topics in ophthalmology, helping students prepare for exams like NEET PG and INI-CET. DigiOne further supports learning by reinforcing the Anatomy of the eye and vision physiology, essential for understanding the underlying causes of Cystoid Macular Edema. Together, these platforms provide comprehensive learning resources to help students navigate CME and other ophthalmological conditions.
Frequently Asked Questions (FAQs)
Q1. What is intraocular lens implantation and how does it improve visual outcome?
Ans. It is a surgical procedure in which a synthetic lens is implanted into the eye to replace the natural lens affected by cataracts. This procedure restores patients’ clear vision by focusing light onto the retina, which results in improving visual outcomes for patients.
Q2. What are some common postoperative complications after uncomplicated cataract surgery?
Ans. Postoperative complications may include temporary discomfort, dry eye symptoms, mild inflammation, and rarely infection. These complications are manageable with postoperative treatment and follow-up care.
Q3. How does diabetes affect cataract surgery and intraocular lens transplantation?
Ans. Cataract surgery outcomes in diabetic patients may influenced by diabetic retinopathy. It requires preoperative assessment, monitoring of retinal thickness, and surgical techniques to achieve optimal results and minimize risks.
Q4. What is the role of subconjunctival injection in postoperative treatment after cataract surgery?
Ans. Subconjunctival injections of medications such as steroids or antibiotics can be used postoperatively to reduce inflammation and prevent infection. It is a localized treatment approach that helps in managing immediate postoperative complications effectively.
Q5. What causes postoperative inflammation after intraocular surgery?
Ans. It includes cataract surgery which is typically triggered by surgical manipulation within the eye. This manipulation can lead to intraocular inflammation characterized by redness, swelling, and discomfort in some patients.
Q6. How common is edema among patients after cataract surgery and what are the risk factors?
Ans. Edema, or swelling of the retina or cornea, can occur after cataract surgery, particularly in patients with diabetes more prone to inflammation. Proper postoperative management including anti-inflammatory treatments, helps mitigate these risks and ensure optimal visual recovery.
Related post